|
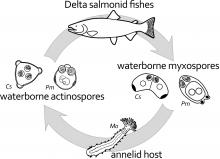
Parvicapsula minibicornis was described in 1996 from adult sockeye salmon (Oncorhynchus nerka) returning to the Fraser River, British Columbia (Kent et al., 1997). Spores of the latter species are present in the tubule lumina and discharged from the urinary bladder. Distinctively, P. minibicornis develops in the glomeruli rather than the renal tubular epithelium as seen in infections by Parvicapsula sp. (Kent et al., 1997; Yasutake and Elliott, 2003). Spores of Parvicapsula sp. are curved and asymmetrical (Hoffman, 1984), whereas spores of P. minibicornis are pyriform and symmetrical (Kent et al., 1997) and do not strictly fit the description of the genus (Shulman, 1966; Lom and Dykova´, 1992). Although these species seem both morphologically and pathologically distinct, closure of the net pen facilities where Parvicapsula sp. was detected has precluded the opportunity to further compare these parasites at a molecular level. Beginning in 1999, infection by P. minibicornis was implicated as a cause of prespawning mortality in wild Fraser River sockeye salmon (St-Hilaire et al., 2002). The parasite was subsequently detected in returning adult pink (Oncorhynchus gorbuscha), coho, and Chinook salmon (Jones et al., 2003) as well as in endangered fish from the Redfish Lake sockeye program in the Columbia River basin, Washington/Oregon/Idaho (Jones et al., 2004). This parasite was first observed in kidneys of juvenile Chinook salmon collected in the Klamath River, Cal-ifornia, in 1999, and subsequent examination of archived tissue samples demonstrated its presence since at least the early 1990s (J. S. Foott, pers. comm.). Its identity as P. minibicornis was confirmed by amplification of a fragment of the small subunit ribosomal RNA gene (18S rDNA) by using the primers of St- Hilaire et al. (2002) (S. R. M. Jones, pers. comm.). The effect of the infection on juvenile salmon populations is not clear, because it is often present concurrently with C. shasta; however, 70–100% of juvenile Chinook salmon are infected during early summer when these fish migrate to the ocean (J. S. Foott, pers. comm.). |
|
Detection Method:
gross pathology / gross clinical signs, microscopic exam - histology, microscopic exam - wet mount Target tissue:
kidney References: |